IMCPL is committed to transforming CGT innovation into clinical reality. By combining biomanufacturing expertise, regulatory compliance, and advanced process control, we help partners de-risk CGT development and accelerate clinical translation.
For partnership opportunities and detailed consultation, contact our Product Development Team today.
IMCPL’s Value Proposition for Industry Partners
- End-to-End CGT Development Expertise:
- Scientific and Technical expertise integrating research, manufacturing, and regulatory strategy.
- Shortest Manufacturing Route
- Cutting-edge process-data analytics ensuring speed and product quality
- Regulatory & Quality Excellence
- Compliance with 21 CFR parts 210 & 211, ensuring regulatory confidence.
- FDA Registered FACT-certified GMP Facility Compliance with 21 CFR parts 210 & 211, ensuring regulatory confidence.
- Regulatory justification, and negotiations with FDA
- Tracking regulatory commitments
- Partnership &Customization
- IMCPL offers tailored solutions aligned with sponsor needs, from small biotech to large pharma.
- Accelerated Timelines
- Streamlined workflows, reducing time-to-IND, bringing therapies to patients faster.
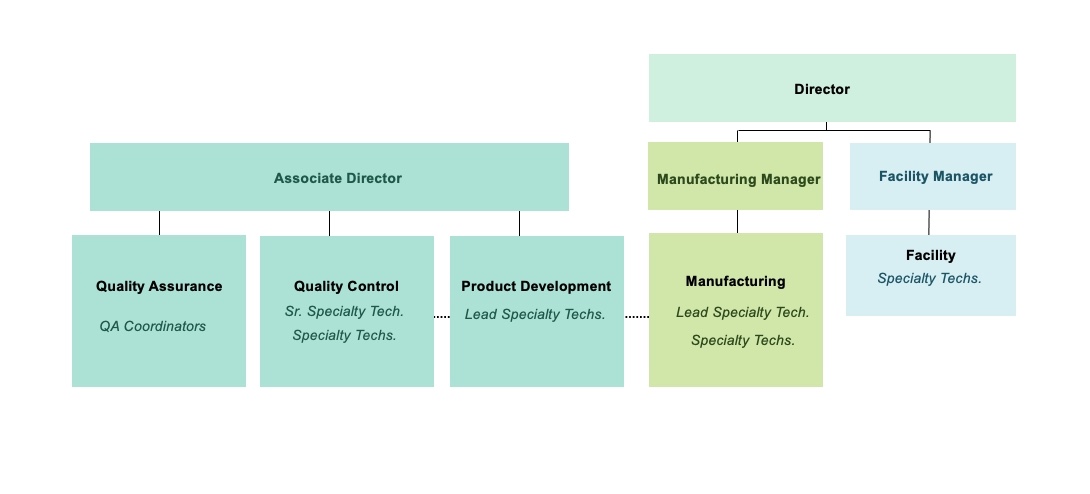
Organization Chart
QC Laboratory Lay Out
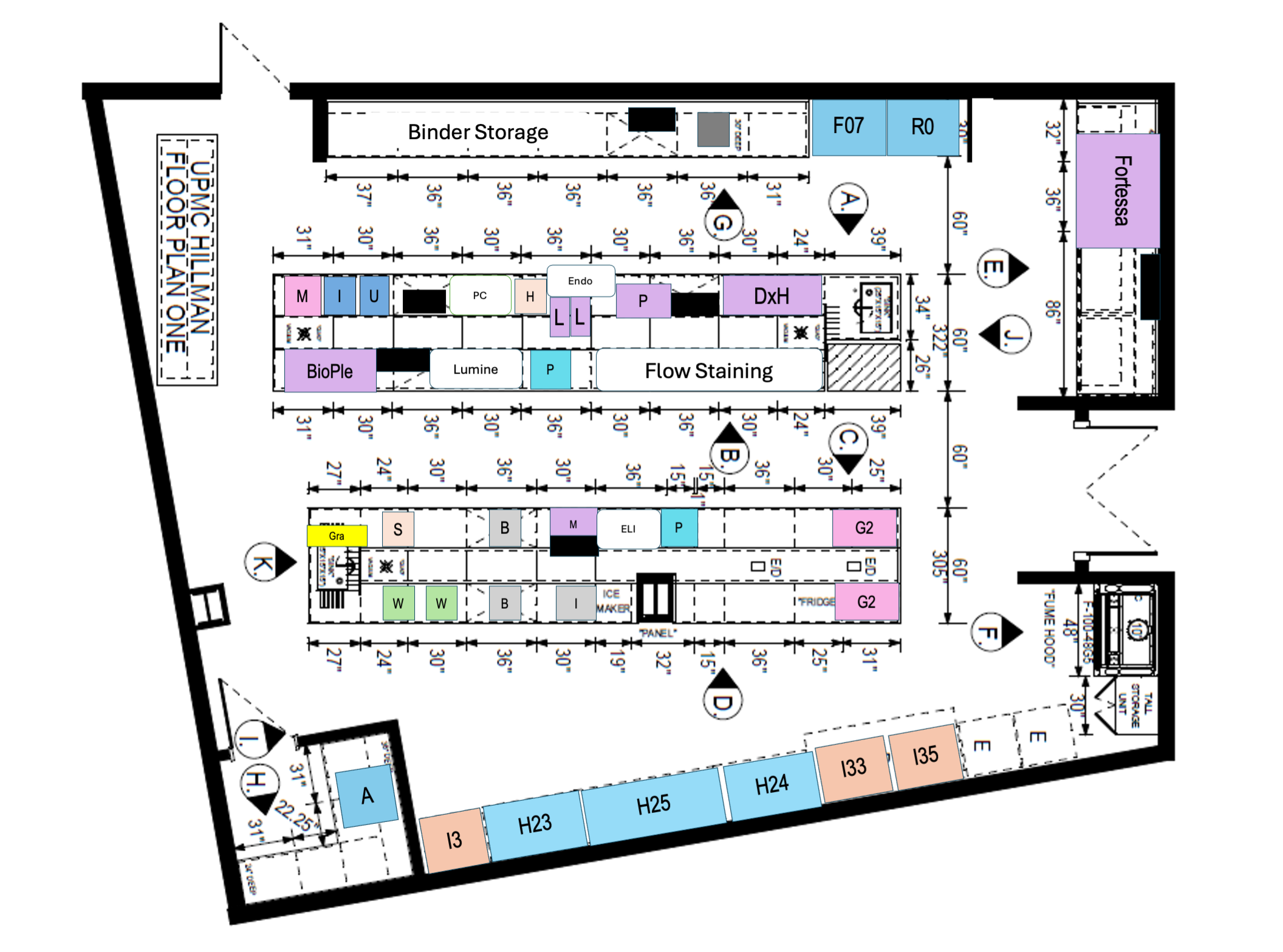
• 2,500 SQ FT
• IND-Testing Volume: 298
• Non-IND Testing Volume: 98
• Tests: Identity, composition, activity/potency, stability, safety testing, Immunomonitoring

