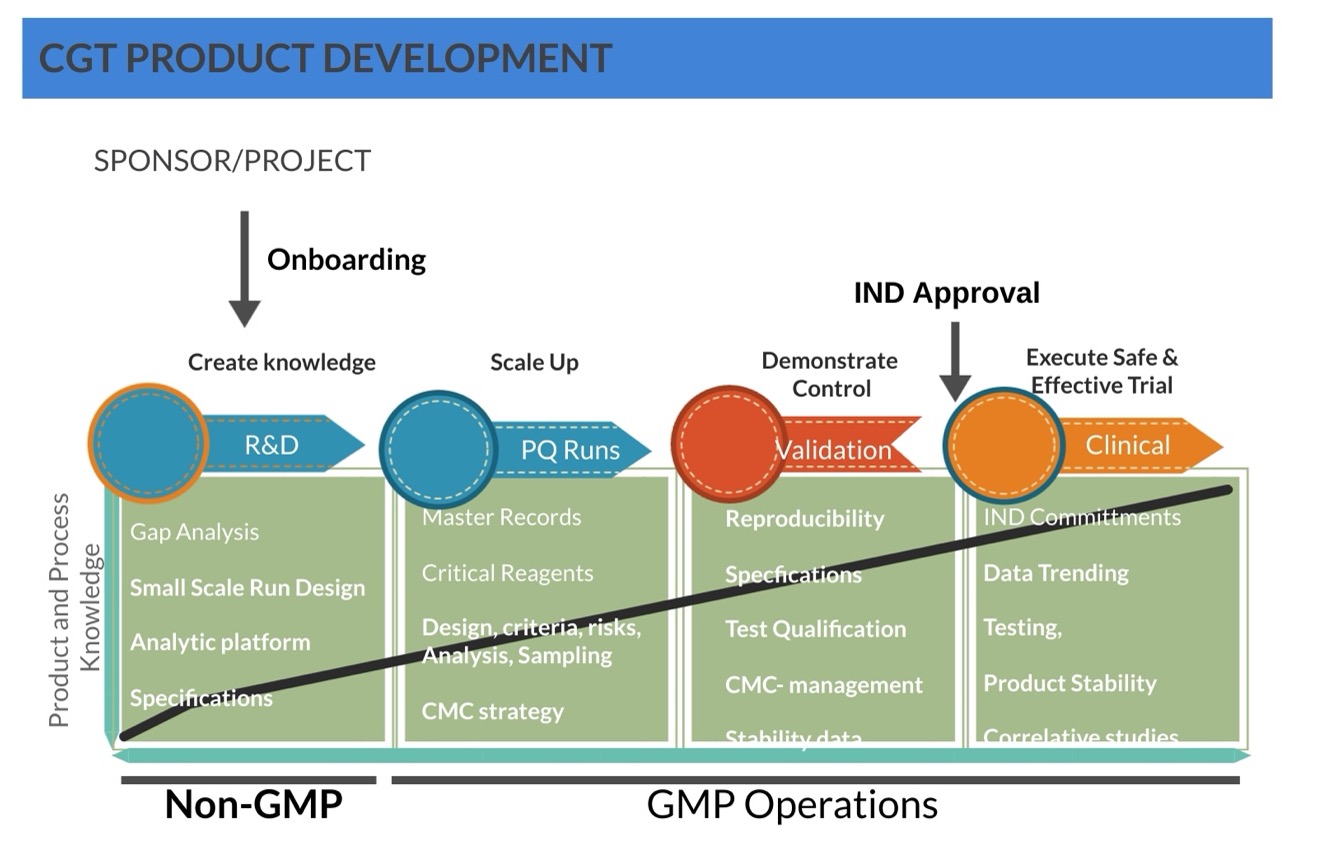
OUR Path to Product Development
Proof of Concept, IND approval, and Clinical Manufacturing
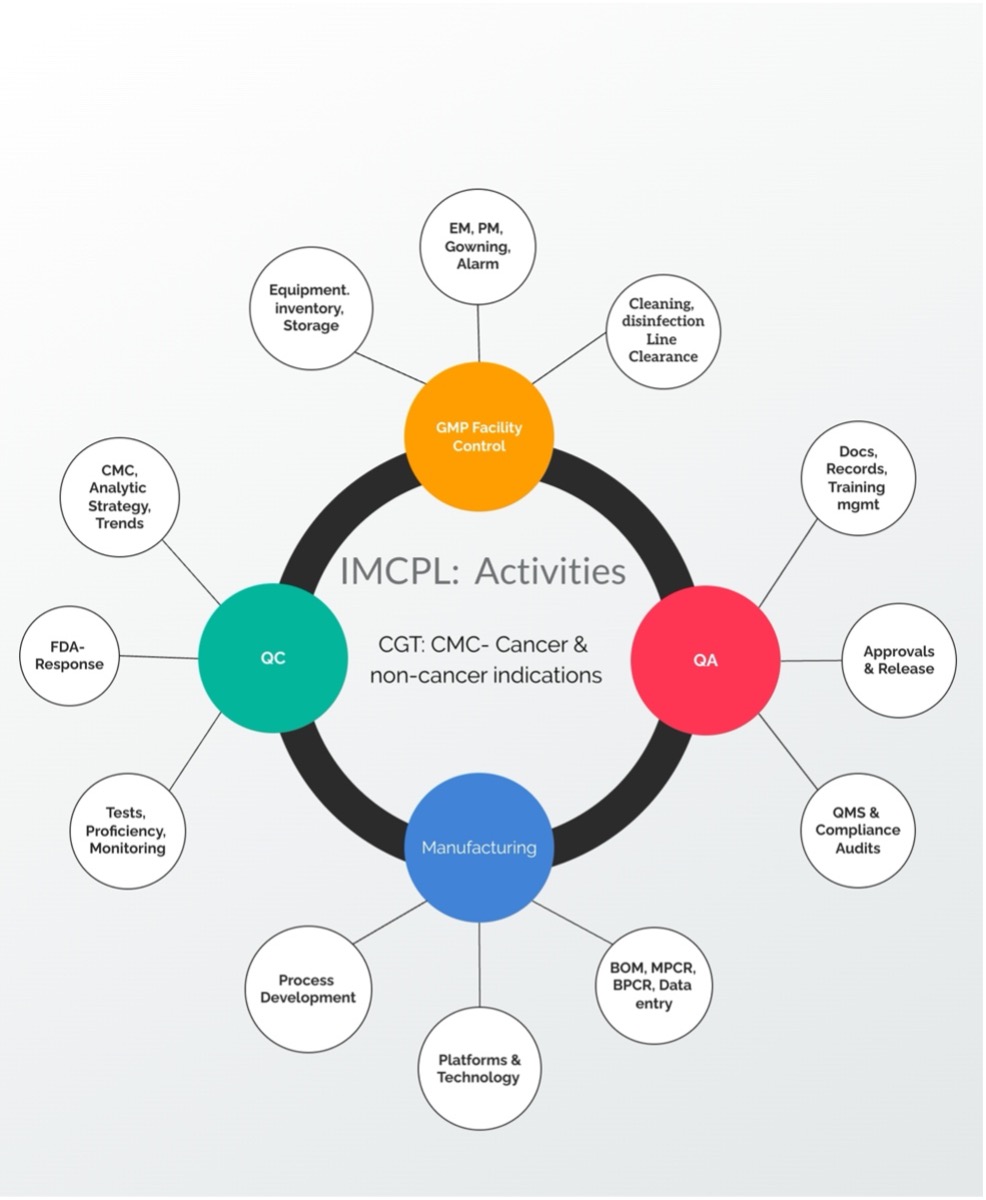
IMCPL is committed to pioneering scalable and reproducible cell and gene therapy (CGT) manufacturing from concept to clinical manufacturing. Our expertise lies in structured project onboarding and in-depth CMC scrutiny, ensuring that process and product quality is well controlled before therapies advance to clinical trials.
Step 1
Project Onboarding & Feasibility Assessment
Goal: Define project scope, feasibility, and alignment with IMCPL's infrastructure and regulatory requirements.
- Internal or External Sponsor Engagement
- Sponsors initiate contact with IMCPL leadership to assess feasibility and strategic fit.
- Involves scope discussions, defining project timelines, and preliminary regulatory and quality assessments.
- Technical and Operational Feasibility Review
- QA, Operations, and Manufacturing teams evaluate resource needs, facility capacity, and material availability.
- Defining Key Success Metrics
- Identification of Target Product Profile (TPP)** and Critical Quality Attributes (CQA).
- Development of a preliminary Quality and Regulatory strategy.
- Regulatory and Financial Structure
- Drafting of Statement of Work (SOW) and budget alignment.
- Establishing Quality and Manufacturing Agreements.
Step 2
Process Development & Optimization
- Technology Transfer to GMP Platform
- R&D finalizes process transfer documentation (Technology Transfer Dossier).
- Pre-GMP audits and equipment calibration to meet regulatory requirements.
Step 3
Process Qualification & Validation (PQR)
Goal: Generate SOPs & Master Production Records. Demonstrate process consistency, reproducibility, and compliance
- Execution of Process Qualification Runs (PQRs)
- Evaluate process scalability and environmental compliance
- Implement Quality adjustments and establish control ranges.
- Final assessment ensuring process homogeneity and reproducibility.
- Analytical & Process Control
- Data tracking and evaluation of key manufacturing parameters.
- Continuous Quality Risk Management based on performance analytics.
- Regulatory Documentation & Stability Testing
- Compilation of process validation reports.
- Short and Long-term stability studies to support regulatory filing.
Step 4
IND Readiness & Regulatory Submission
Goal: Generate comprehensive data to support an IND application.
- Final Validation Runs
- Execution of GMP-validated production batches.
- Preparation of lot release specifications and regulatory-compliant reports.
- Preparation of IND Submission Package
- CMC (Chemistry, Manufacturing, and Controls) data compilation.
- Regulatory meetings to align with FDA expectations.
- Regulatory Filing & Review Process
- Submission of IND application, followed by FDA review cycles.
- Addressing regulatory queries and implementing process improvements

